The molecules are synthesized from cost-effective isotope sources such as ¹³CH₃I, D₂O, ¹³C-acetone, and ¹³C-formaldehyde. We developed these compounds and used them in various overexpression systems to achieve highly selective isotope incorporation at target residues. The resulting protein samples are tailored to study protein dynamics, structure and interaction by NMR spectroscopy. This selection presented here focuses primarily on side-chain labeling, although other compounds are also employed for backbone labeling.
Alanine, Isoleucine, Valine and Leucine labeling
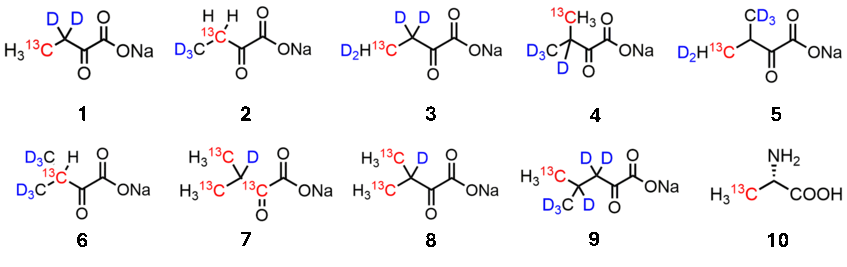
Isotopologues of alpha-Ketobutyrate (1-3) serve as effective Isoleucine precursors in E. coli overexpression media. Alpha-Ketoisovalerates (4-8) label Valine and Leucine side chains. We developed methods to separate Leucine from Valine labeling by introducing a novel Leucine precursor alpha-Ketoisocaproate (9).
Synthesis of these compounds and examples of their application can be found at J. Am. Chem. Soc. 2004, 5348; Biochemistry 2006, 8885; J. Biomol. NMR 2008, 111; ChemBioChem 2013, 818; J. Biomol NMR 2013, 205; J. Biomol NMR 2023, 131; J. Biomol. NMR 2023, 1; ChemBioChem 2024, e202300762; J. Med. Chem. 2024, 13187; J. Biomol NMR 2024, 237.
Methionine labeling
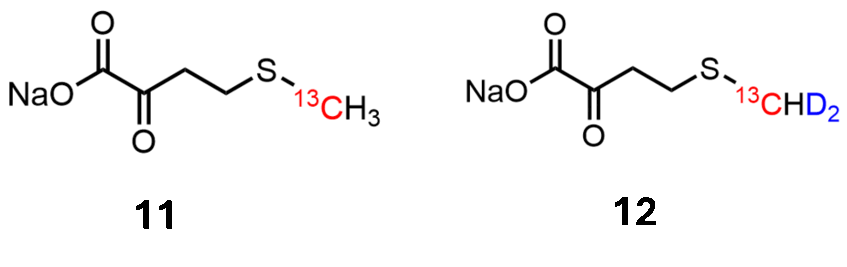
Methionine labeling
The alpha-ketoacids 11 and 12 are highly selective precursors for methyl labeling in Methionine side-chains (ChemBioChem 2007, 610; J. Biomol. NMR 2007, 125)
Phenylalanine and Tyrosine labeling
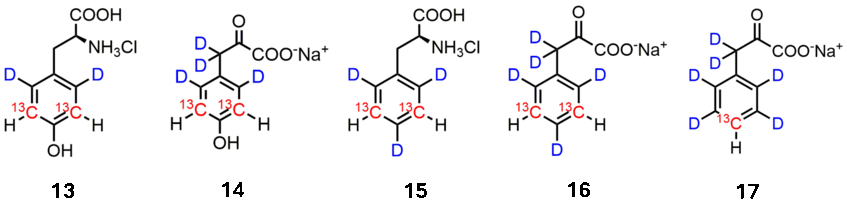
Isolated 13C-H spin systems in otherwise deuterated side chains are key to study dynamic processes (eg. ring flips) of the target residues or study their role at interaction surfaces in detail. We could show that alpha-Ketoacids are effectively taken up and metabolized to the corresponding amino acids Phe and Tyr. 13C is introduced in the synthesis of these compounds by using isotopologues of acetone to create the aromatic system. This approach allows for installing C-13 at meta (13-16) or para positions (17).
Synthesis of these compounds and applications: J. Biomol. NMR 2013, 327; Org. Biomol. Chem. 2014, 7551; J. Biomol. NMR 2018, 129; J. Am. Chem. Soc. 2019, 11183; J. Biomol. NMR 2019, 633; J. Biomol. NMR 2021, 383; J. Struct. Biol. X 2023, 100079; Angew. Chem. Int. Ed. 2023, e202219314)
Tryptophan labeling
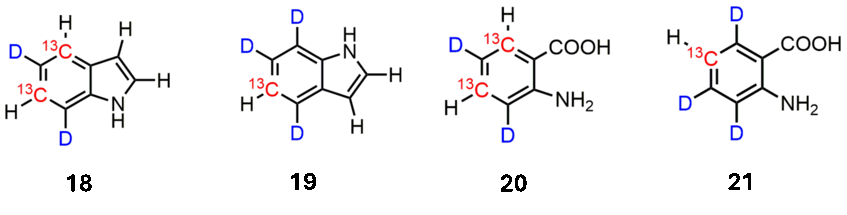
The indole side chain of tryptophan is frequently found at interaction sites of important target proteins. Addition of simple precursors, such as indoles 18 and 19, as well as anthranilic acids 20 and 21, are important additives in the minimal medium of overexpressing microorganisms to create defined isotope patterns of Trp-residues. The highly selective deuteration pattern is created by using D2O at elevated temperatures with activated aryl intermediates.
Publications on Trp labeling: ChemBioChem 2015, 746; J. Biomol. NMR 2017, 13; J. Biomol. NMR 2018, 129; Angew. Chem. Int. Ed. 2020, 14861.
Histidine labeling
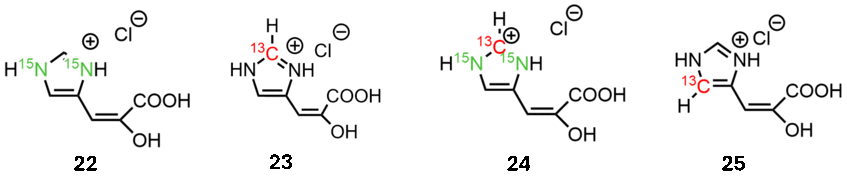
The importance of histidine extends far beyond its role as an acid-base catalyst in protease catalytic triads. We identified a non-chiral compound, that enables selective labeling of this target residue. Moe recently, we developed synthetic approaches to generate additional isotopologues 22-25, which can be used to study for example the charge state of the imidazole side chain.
Publications related to this topic: ChemBioChem 2017, 1487; Biophys. J. 2024, 1542; J. Mol. Biol. accepted manuscript.
13C-19F Labeling
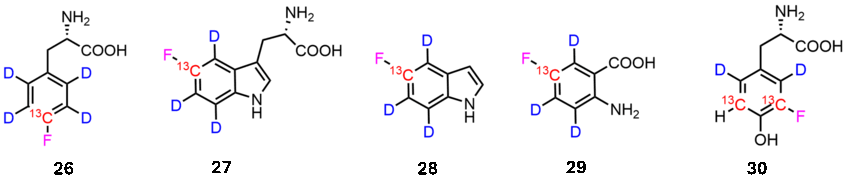
Fluorine is a very sensitive and bioorthogonal reporter in biomolecular NMR spectroscopy. We introduced methods, which combine fluorination protocols with deuteration techniques to create isolated 13C-19F systems in the aryl side-chains of Phe, Tyr and Trp. These compounds can be used not only in E. coli expression systems, but also in mammalian cell lines. The resulting fluorinated proteins represent ideal samples to record for example CF-TROSY experiments.
Key publications on this topic: J. Biomol. NMR 2024, 139; Chem. Comm. 2024, 14188.
Arginine Labeling
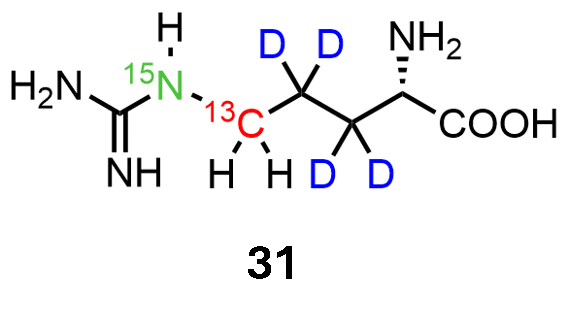
Arginine Labeling
Compound 31 was developed to use arginines Cdelta in combination with Nepsilon to study protein interaction by NMR. Being an amino acid instead of a metabolic precursor, this compound can be used in Cell-free approaches, as well as cell-based expression protocols.
Synthesis and application reported in Chem. Eur. J. 2025, e202400663.
Phosphotyrosine labeling
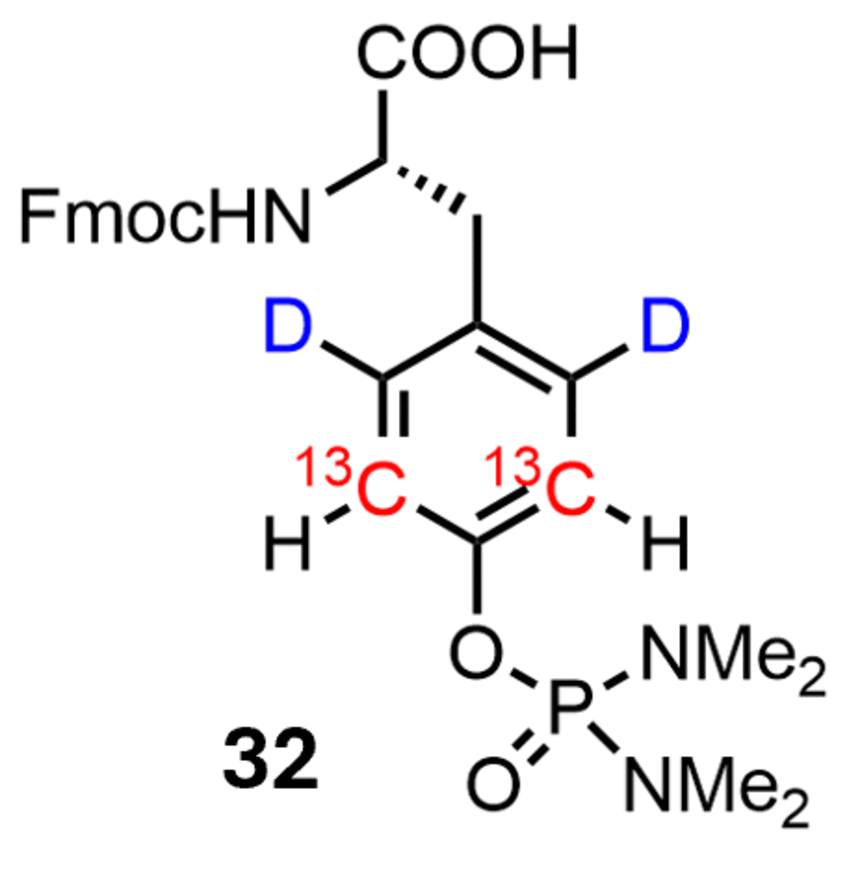
Phosphotyrosine labeling
The synthesis of this compound provides a building block for solid-phase peptide synthesis, enabling the preparation of peptides containing phosphotyrosine (pTyr). These peptides can then be used to probe interactions with protein binding partners, facilitating analysis of the role of phosphorylation in regulating these interactions.
Published in ChemBioChem 2025, e202400663.
